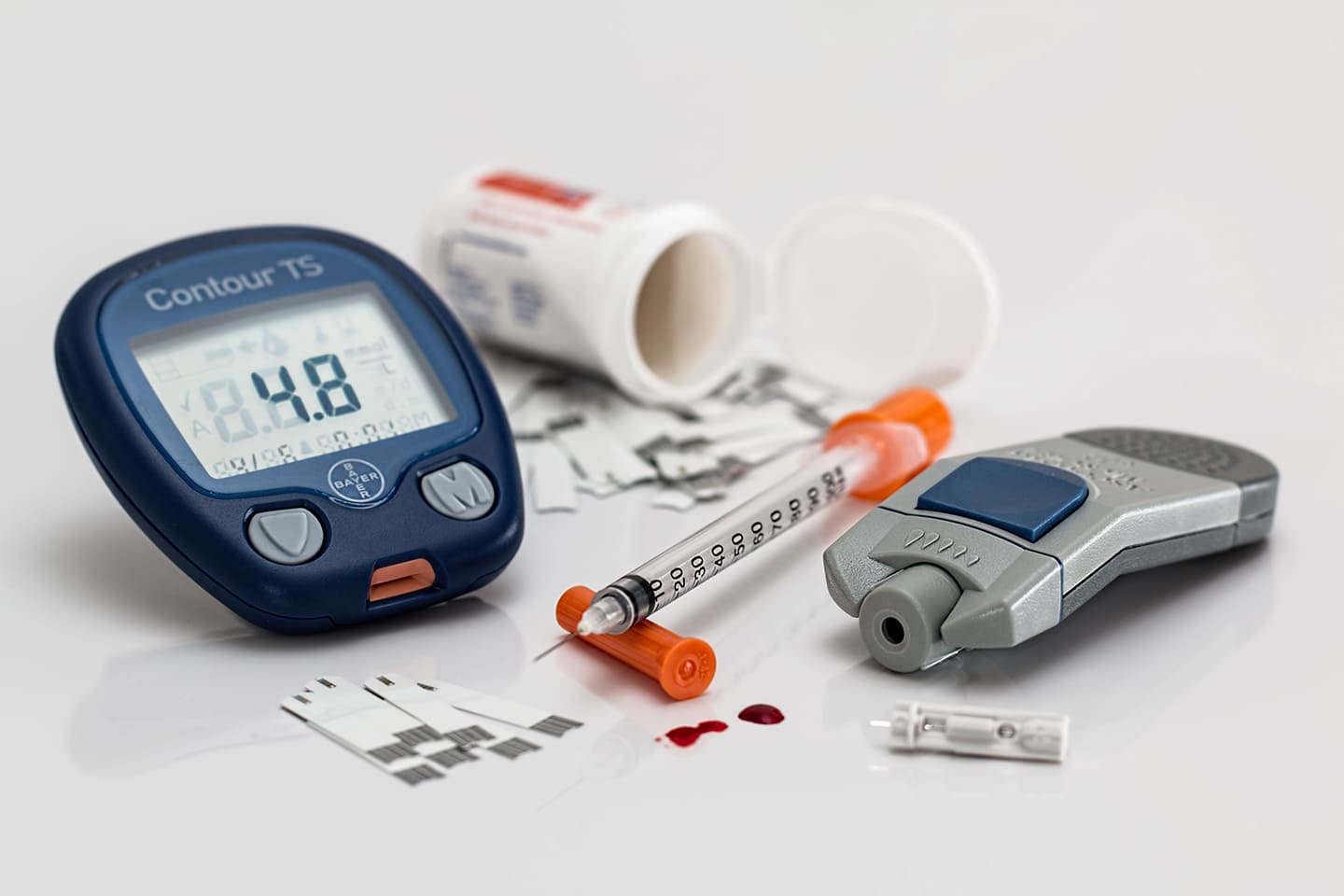
The Medical Device industry is very broad. By definition, it is an apparatus, implant or in vitro reagent that is used to diagnose, prevent or treat disease or other conditions without the use of a chemical action within or on the body. Medical devices can vary in their complexity. Examples range from tongue depressors, bandages to blood glucose meters to pacemakers.